in celiac disease (DC), O gluten triggers an autoimmune process that leads to damage to the small intestine mucosa and, currently, there is no medicine for the celiac disease, just totally gluten free diet.
From studies, the COUR group (professional scientists seeking to find treatments and cures for immune-mediated diseases), developed a nanoparticle capable of causing tolerance to gluten in the celiac's body.
A Takeda Pharmaceutical Company, a pharmaceutical company from Japan, following COUR's disclosure of its success in creating the new nanoparticle, demonstrated its interests and bought the rights to the medication., starting clinical studies.
How does the CNP-101/TAK-101 celiac disease drug work
A CNP-101 (TIMP-GLIA) it is a biodegradable nanoparticle that encapsulates the gliadin protein – main triggering component of CD. This nanoparticle acts as a “Trojan Horse”, hiding the allergen inside the capsule, in order to convince the immune system not to attack it.
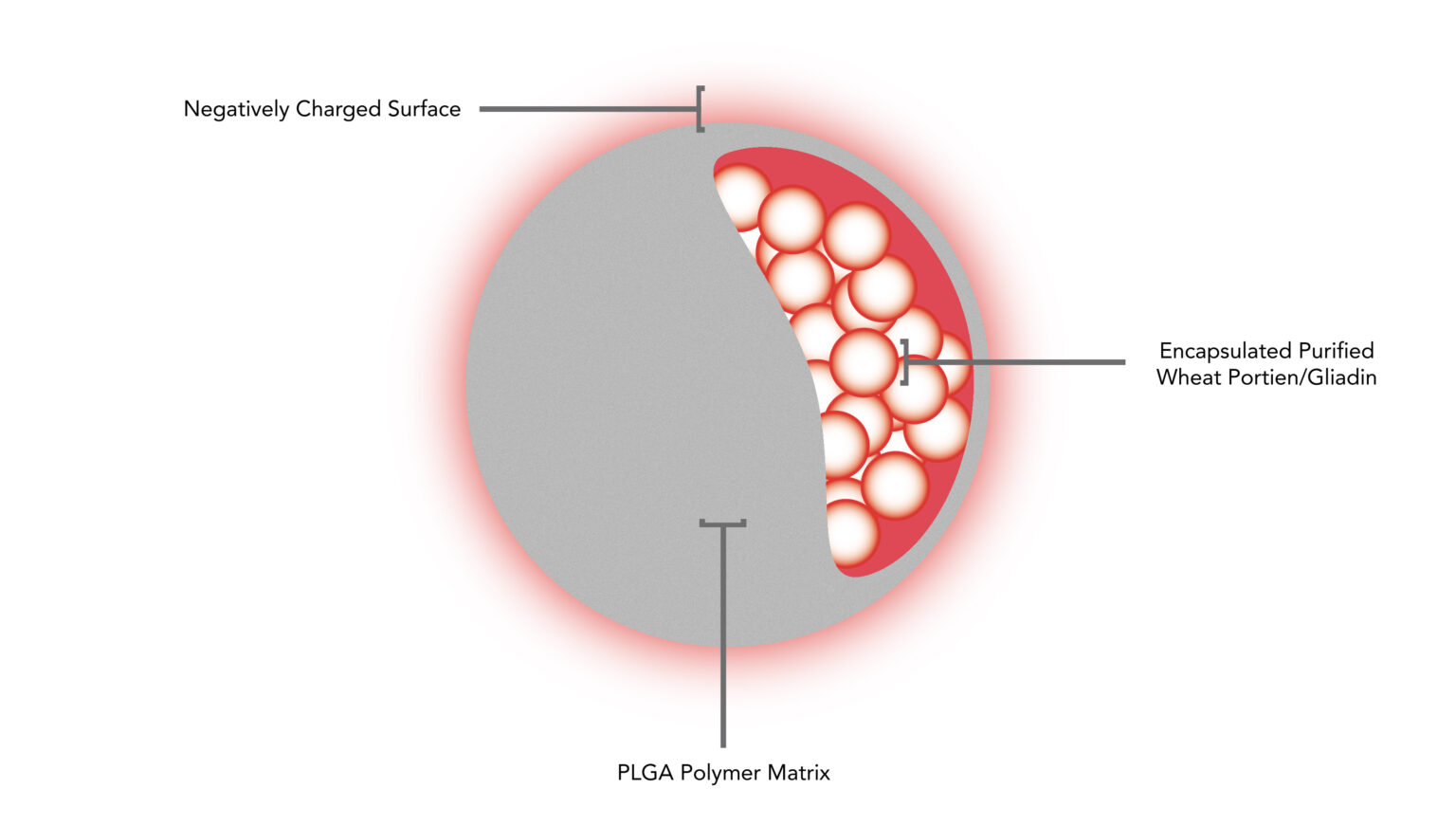
When the allergen-laden nanoparticle is injected into the bloodstream, the immune system is not worried about her, for you see it as harmless debris. Naturally the nanoparticle is consumed by a macrophage (body defense cell). As a PLGA capsule – poly(lactic acid-co-glycolic acid) is made of a highly biodegradable and biocompatible material., and the allergen is “escondido”, the immune system turns off the attack and is reset to its normal performance..
In addition to celiac disease, the technology could potentially handle a number of other diseases and allergies, including multiple sclerosis, type diabetes 1, peanut allergy, asthma and more. The technology was developed in the laboratory of theDr. Stephen Miller, professor of microbiology and immunology at Northwestern University.
So far the medication has been tested in a randomized, double-blind Phase 2 to assess the efficacy and safety of CNP-101 in the dosage of 8 mg/kg versus placebo in celiac patients.
Curiosity: what do you do in a phase 2 of clinical study?
study phase 2 normally encompasses: from 100 a 300 individuals who have the disease for which the drug is being studied. The goal is to get more safety data and start evaluating the effectiveness of the new drug. They usually test different dosages and indications.
UNICAMP – Faculty of Middle Sciences
The study
The gluten challenge has begun 7 days after the administration of two test dosages for “deactivation” of the immune system. The treatment included 12 g of gluten per day for 3 days in a row and later, 6 g/day of gluten during 11 days. Endoscopy was performed before treatment and at the end of the gluten challenge.
A total of 34 patients were randomized and treated; six discontinued the study because of symptoms related to gluten and 28 completed the full protocol of 14 days. No patient had significant clinical changes in vital signs, routine laboratory tests or cytokines / serum chemokines, proliferation of gliadin-specific T cells and cytokine secretion. The complement levels (complex of defense cells that participate in innate and acquired defenses) increased plasma levels in most patients; Nonetheless, this transient effect was not reflected in associated adverse events.
The most frequent adverse events observed in patients treated with CNP-101 with the corresponding value in parentheses for patients treated with placebo were: nausea 81% (72%), abdominal distension 56% (61%), diarrhea 50% (50%), headache 44% (17%), abdominal pain 38% (28%), abnormal gastrointestinal sounds 38% (39%), vomiting 31% (33%), fatigue 31% (50%) and back pain 31% (0).
Comparing the intervention and placebo groups:
Comparing intervention group and placebo group, the intervention group obtained 4 more aggravated symptoms versus 4 symptoms in the placebo group and 1 tie. Although the percentage of symptoms seems scary, the purpose of the study was successfully completed. Besides, the placebo group showed a reduction in the height of the intestinal villi in the endoscopy performed after the end of the study.
Finally, the study showed that CNP-101 medication was able to prevent the immune activation induced by gluten ingestion in adults with CD. As far as we know, this is the first clinical trial to demonstrate non-autologous induction of antigen-specific immune tolerance in any autoimmune disease.
And there, would you take this drug for celiac disease if it was approved?? tell me in the comments!
See here the original article on the celiac disease drug
Late breaking abstracts (wiley.com) Available on the page 11
Excerpts Takeda format (courpharma.com)
